Page 140 - Science Course 2 (Book 1)
P. 140
Mo5-L2b : What is a Polar Molecule?
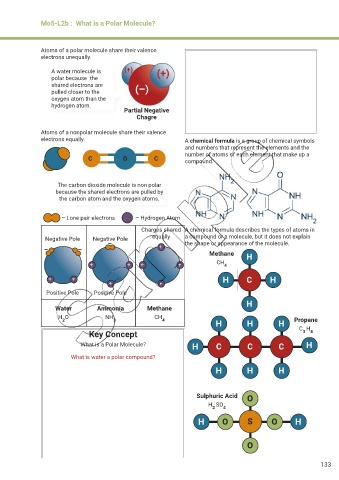
Atoms of a polar molecule share their valence
electrons unequally.
A water molecule is (+) (+)
polar because the
shared electrons are (–)
pulled closer to the
oxygen atom than the
hydrogen atom.
Partial Negative
Chagre
Atoms of a nonpolar molecule share their valence
electrons equally. A chemical formula is a group of chemical symbols
and numbers that represent the elements and the
number of atoms of each element that make up a
C O C compound.
The carbon dioxide molecule is non polar
because the shared electrons are pulled by
the carbon atom and the oxygen atoms.
– Lone pair electrons – Hydrogen Atom
Charges shared A chemical formula describes the types of atoms in
Negative Pole Negative Pole equally a compound or a molecule, but it does not explain
– the shape or appearance of the molecule.
– – +
Methane H
+ + + + CH 4
+ + H C H
+ +
Positive Pole Positive Pole
H
Water Ammonia Methane
H O NH 3 CH 4 Propane
2
H H H
Key Concept C H
3 8
What is a Polar Molecule? H C C C H
What is water a polar compound?
H H H
Sulphuric Acid O
H SO 4
2
H O S O H
O
133