Page 112 - Science Course 2 (Book 1)
P. 112
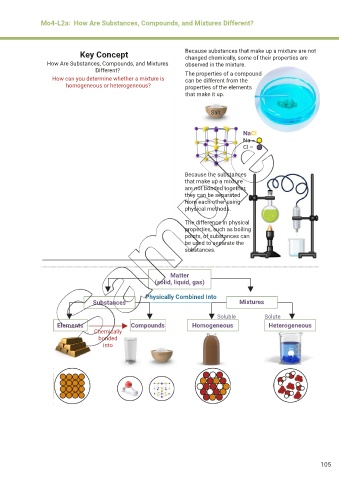
Mo4-L2a: How Are Substances, Compounds, and Mixtures Different?
Key Concept Because substances that make up a mixture are not
changed chemically, some of their properties are
How Are Substances, Compounds, and Mixtures observed in the mixture.
Different? The properties of a compound
How can you determine whether a mixture is can be different from the
homogeneous or heterogeneous? properties of the elements
that make it up.
Salt
NaCl
Na –
Cl –
Because the substances
that make up a mixture
are not bonded together,
they can be separated
from each other using
physical methods.
The difference in physical
properties, such as boiling
points, of substances can
be used to separate the
substances.
Matter
(solid, liquid, gas)
Physically Combined Into
Substances Mixtures
Soluble Solute
Elements Compounds Homogeneous Heterogeneous
Chemically
bonded
into
105