Page 113 - Science Course 2 (Book 1)
P. 113
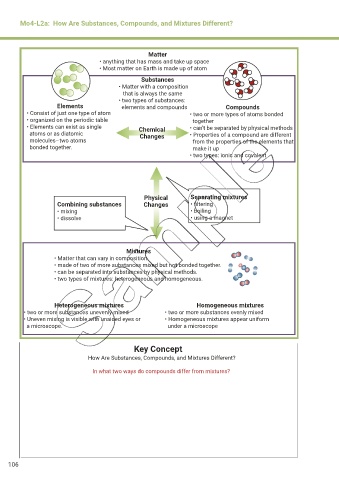
Mo4-L2a: How Are Substances, Compounds, and Mixtures Different?
Matter
• anything that has mass and take up space
• Most matter on Earth is made up of atom
Substances
• Matter with a composition
that is always the same
• two types of substances:
Elements elements and compounds Compounds
• Consist of just one type of atom • two or more types of atoms bonded
• organized on the periodic table together
• Elements can exist as single Chemical • can’t be separated by physical methods
atoms or as diatomic Changes • Properties of a compound are different
molecules–two atoms from the properties of the elements that
bonded together. make it up
• two types: ionic and covalent
Physical Separating mixtures
Combining substances Changes • filtering
• mixing • boiling
• dissolve • using a magnet
Mixtures
• Matter that can vary in composition.
• made of two of more substances mixed but not bonded together.
• can be separated into substances by physical methods.
• two types of mixtures: heterogeneous and homogeneous.
Heterogeneous mixtures Homogeneous mixtures
• two or more substances unevenly mixed • two or more substances evenly mixed
• Uneven mixing is visible with unaided eyes or • Homogeneous mixtures appear uniform
a microscope. under a microscope
Key Concept
How Are Substances, Compounds, and Mixtures Different?
In what two ways do compounds differ from mixtures?
106