Page 138 - Science Course 2 (Book 1)
P. 138
Mo5-L2a: What are Covalent Compounds? Mo5-L2a: What are Covalent Compounds?
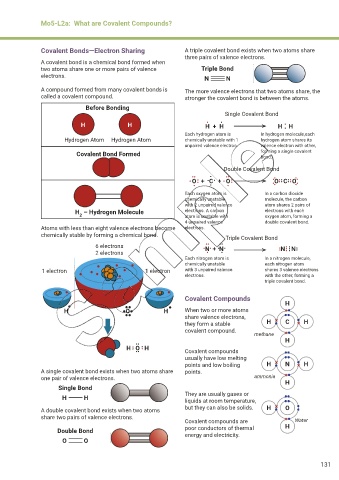
Covalent Bonds—Electron Sharing A triple covalent bond exists when two atoms share
three pairs of valence electrons.
A covalent bond is a chemical bond formed when
two atoms share one or more pairs of valence Triple Bond
electrons. N N
A compound formed from many covalent bonds is The more valence electrons that two atoms share, the
called a covalent compound. stronger the covalent bond is between the atoms.
Before Bonding
Single Covalent Bond
•
•
H H H + H H H
•
•
Each hydrogen atom is In hydrogen molecule,each
Hydrogen Atom Hydrogen Atom chemically unstable with 1 hydrogen atom shares its
unpaired valence electron. valence electron with other,
Covalent Bond Formed forming a single covalent
bond.
Double Covalent Bond
•• • ••
• O + C + O •• • • • • • O C O •• ••
••••
••
••
• • • •• ••
Each oxygen atom is In a carbon dioxide
chemically unstable molecule, the carbon
with 2 unpaired valence atom shares 2 pairs of
H – Hydrogen Molecule electrons. A carbon electrons with each
2
atom is unstable with
oxygen atom, forming a
4 unpaired valence double covalent bond.
Atoms with less than eight valence electrons become electrons.
chemically stable by forming a chemical bond. Triple Covalent Bond
6 electrons •• •• •••
•••
N N ••
2 electrons • • • • • ••
N + N•
Each nitrogen atom is In a nitrogen molecule,
chemically unstable each nitrogen atom
1 electron 1 electron with 3 unpaired valence shares 3 valence electrons
electrons. with the other, forming a
triple covalent bond.
Covalent Compounds
H
H O H When two or more atoms
share valence electrons,
they form a stable H C H
covalent compound.
methane
H
H O H
••
•• Covalent compounds
•• ••
usually have low melting
points and low boiling H N H
A single covalent bond exists when two atoms share points.
one pair of valence electrons. ammonia H
Single Bond
H H They are usually gases or
liquids at room temperature,
A double covalent bond exists when two atoms but they can also be solids. H O
share two pairs of valence electrons.
Covalent compounds are Water
Double Bond poor conductors of thermal H
energy and electricity.
O O
131