Page 101 - Science Course 2 (Book 1)
P. 101
Concentration and Solubility Mo4-L1a: What are the Properties of a Solution?
Lesson 1
Let’s Begin
In this topic, you will learn the following
lessons: Parts of Solutions
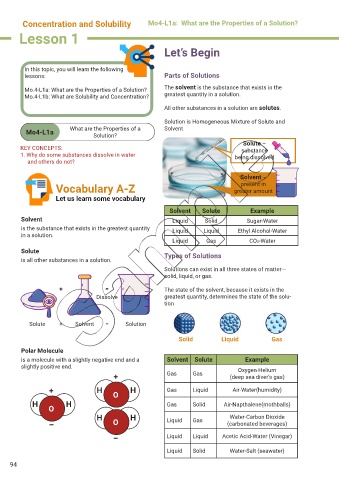
Mo.4-L1a: What are the Properties of a Solution? The solvent is the substance that exists in the
Mo.4-L1b: What are Solubility and Concentration? greatest quantity in a solution.
All other substances in a solution are solutes.
Solution is Homogeneous Mixture of Solute and
Mo4-L1a What are the Properties of a Solvent
Solution?
Solute –
KEY CONCEPTS: substance
1. Why do some substances dissolve in water being dissolved
and others do not?
Solvent –
present in
Vocabulary A-Z greater amount
Let us learn some vocabulary
Solvent Solute Example
Solvent Liquid Solid Sugar-Water
is the substance that exists in the greatest quantity Liquid Liquid Ethyl Alcohol-Water
in a solution.
Liquid Gas CO2-Water
Solute Types of Solutions
is all other substances in a solution.
Solutions can exist in all three states of matter—
solid, liquid, or gas.
+ = The state of the solvent, because it exists in the
Dissolve greatest quantity, determines the state of the solu-
tion
Solute + Solvent = Solution
Solid Liquid Gas
Polar Molecule
is a molecule with a slightly negative end and a Solvent Solute Example
slightly positive end. Oxygen-Helium
+ Gas Gas (deep sea diver’s gas)
+ H O H Gas Liquid Air-Water(humidity)
H H Gas Solid Air-Napthalene(mothballs)
O
H H Liquid Gas Water-Carbon Dioxide
– O (carbonated beverages)
– Liquid Liquid Acetic Acid-Water (Vinegar)
Liquid Solid Water-Salt (seawater)
94