Page 103 - Science Course 2 (Book 1)
P. 103
Mo4-L1a: What are the Properties of a Solution?
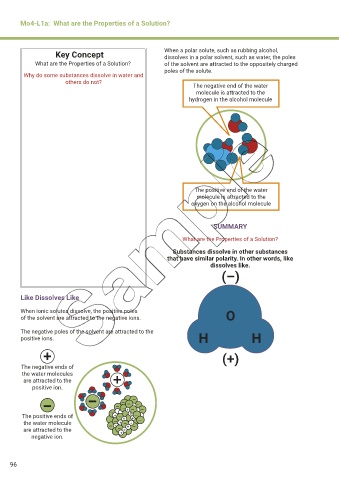
Key Concept When a polar solute, such as rubbing alcohol,
dissolves in a polar solvent, such as water, the poles
What are the Properties of a Solution? of the solvent are attracted to the oppositely charged
poles of the solute.
Why do some substances dissolve in water and
others do not?
The negative end of the water
molecule is attracted to the
hydrogen in the alcohol molecule
The positive end of the water
molecule is attracted to the
oxygen on the alcohol molecule
SUMMARY
What are the Properties of a Solution?
Substances dissolve in other substances
that have similar polarity. In other words, like
dissolves like.
(–)
Like Dissolves Like
When ionic solutes dissolve, the positive poles O
of the solvent are attracted to the negative ions.
The negative poles of the solvent are attracted to the
positive ions. H H
(+)
The negative ends of
the water molecules
are attracted to the
positive ion.
–
– – –
–
– – + – –
The positive ends of + – + + + –
the water molecule – – + – + –
+
are attracted to the – + + –
+
negative ion.
96