Page 106 - Science Course 2 (Book 1)
P. 106
Mo4-L1b: What are Solubility and Concentration?
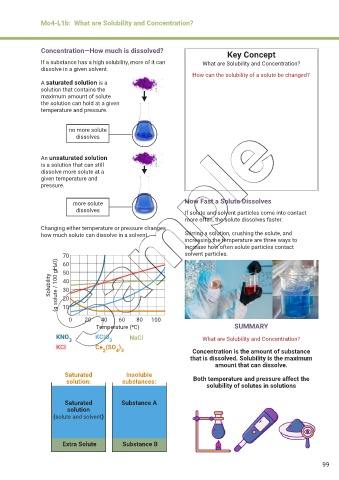
Concentration—How much is dissolved? Key Concept
If a substance has a high solubility, more of it can What are Solubility and Concentration?
dissolve in a given solvent.
How can the solubility of a solute be changed?
A saturated solution is a
solution that contains the
maximum amount of solute
the solution can hold at a given
temperature and pressure.
no more solute
dissolves
An unsaturated solution
is a solution that can still
dissolve more solute at a
given temperature and
pressure.
more solute How Fast a Solute Dissolves
dissolves If solute and solvent particles come into contact
more often, the solute dissolves faster.
Changing either temperature or pressure changes
how much solute can dissolve in a solvent. Stirring a solution, crushing the solute, and
increasing the temperature are three ways to
increase how often solute particles contact
70 solvent particles.
60
Solubility (g solute / 100 gH20) 50
40
30
20
10
0 20 40 60 80 100
Temperature (0C) SUMMARY
KNO 3 KCIO 3 NaCl What are Solubility and Concentration?
KCI Ce (SO ) Concentration is the amount of substance
4 3
2
that is dissolved. Solubility is the maximum
amount that can dissolve.
Saturated Insoluble
solution: substances: Both temperature and pressure affect the
solubility of solutes in solutions
Saturated Substance A
solution
(solute and solvent)
Extra Solute Substance B
99