Page 127 - Science Course 2 (Book 1)
P. 127
Atomic Bonds Mo5-L1a: Where Do Electrons Have the Most
Energy?
Lesson 1
Let’s Begin
In this topic, you will learn the following
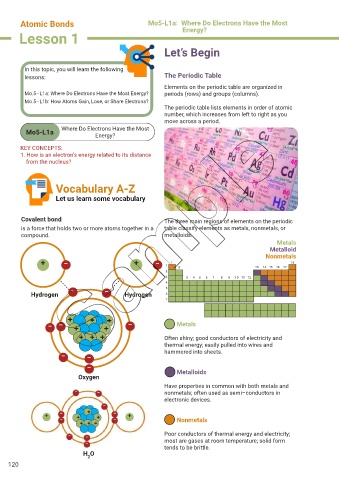
lessons: The Periodic Table
Elements on the periodic table are organized in
Mo.5–L1a: Where Do Electrons Have the Most Energy? periods (rows) and groups (columns).
Mo.5–L1b: How Atoms Gain, Lose, or Share Electrons?
The periodic table lists elements in order of atomic
number, which increases from left to right as you
move across a period.
Mo5-L1a Where Do Electrons Have the Most
Energy?
KEY CONCEPTS:
1. How is an electron’s energy related to its distance
from the nucleus?
Vocabulary A-Z
Let us learn some vocabulary
Covalent bond The three main regions of elements on the periodic
is a force that holds two or more atoms together in a table classify elements as metals, nonmetals, or
compound. metalloids.
Metals
Metalloid
Nonmetals
+ – + –
– –
Hydrogen Hydrogen
– – + + + + – Metals
+
Often shiny; good conductors of electricity and
+
+
+
thermal energy; easily pulled into wires and
hammered into sheets.
– –
–
Oxygen Metalloids
Have properties in common with both metals and
nonmetals; often used as semi–conductors in
electronic devices.
Nonmetals
Poor conductors of thermal energy and electricity;
most are gases at room temperature; solid form
tends to be brittle.
H O
2
120