Page 132 - Science Course 2 (Book 1)
P. 132
Mo5-L1b: How Atoms Gain, Lose, or Share Electrons?
Atoms gain, lose, or share valence electrons and SUMMARY
become chemically stable.
How Atoms Gain, Lose, or Share Electrons?
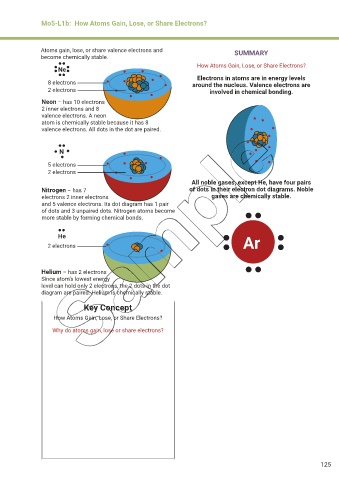
Ne
Electrons in atoms are in energy levels
8 electrons around the nucleus. Valence electrons are
2 electrons involved in chemical bonding.
Neon – has 10 electrons
2 inner electrons and 8
valence electrons. A neon
atom is chemically stable because it has 8
valence electrons. All dots in the dot are paired.
N
5 electrons
2 electrons
All noble gases, except He, have four pairs
Nitrogen – has 7 of dots in their electron dot diagrams. Noble
electrons 2 inner electrons gases are chemically stable.
and 5 valence electrons. Its dot diagram has 1 pair
of dots and 3 unpaired dots. Nitrogen atoms become
more stable by forming chemical bonds.
He
2 electrons Ar
Helium – has 2 electrons
Since atom’s lowest energy
level can hold only 2 electrons, the 2 dots in the dot
diagram are paired. Helium is chemically stable.
Key Concept
How Atoms Gain, Lose, or Share Electrons?
Why do atoms gain, lose or share electrons?
125