Page 129 - Science Course 2 (Book 1)
P. 129
Mo5-L1a: Where Do Electrons Have the Mo5-L1b: How Atoms Gain, Lose, or Share
Most Energy? Electrons?
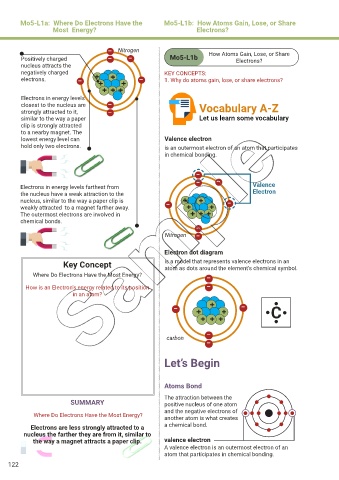
– Nitrogen How Atoms Gain, Lose, or Share
Positively charged – – Mo5-L1b Electrons?
nucleus attracts the
negatively charged KEY CONCEPTS:
electrons. – + + + – 1. Why do atoms gain, lose, or share electrons?
+
Electrons in energy levels + + +
closest to the nucleus are – Vocabulary A-Z
strongly attracted to it, –
similar to the way a paper Let us learn some vocabulary
clip is strongly attracted
to a nearby magnet. The
lowest energy level can Valence electron
hold only two electrons. is an outermost electron of an atom that participates
in chemical bonding.
–
– – Valence
Electrons in energy levels farthest from
the nucleus have a weak attraction to the Electron
nucleus, similar to the way a paper clip is – + + –
weakly attracted to a magnet farther away. + +
The outermost electrons are involved in + + +
chemical bonds.
–
Nitrogen –
Electron dot diagram
Key Concept is a model that represents valence electrons in an
atom as dots around the element’s chemical symbol.
Where Do Electrons Have the Most Energy? –
How is an Electron’s energy related to its position –
in an atom?
C
– + –
+
+
+
+
+
–
carbon
–
Let’s Begin
Atoms Bond
The attraction between the
SUMMARY positive nucleus of one atom
and the negative electrons of
Where Do Electrons Have the Most Energy?
another atom is what creates
Electrons are less strongly attracted to a a chemical bond.
nucleus the farther they are from it, similar to
the way a magnet attracts a paper clip. valence electron
A valence electron is an outermost electron of an
atom that participates in chemical bonding.
122